Heidi Kaastrup Müller - Molecular Neuroscience
Research Aim
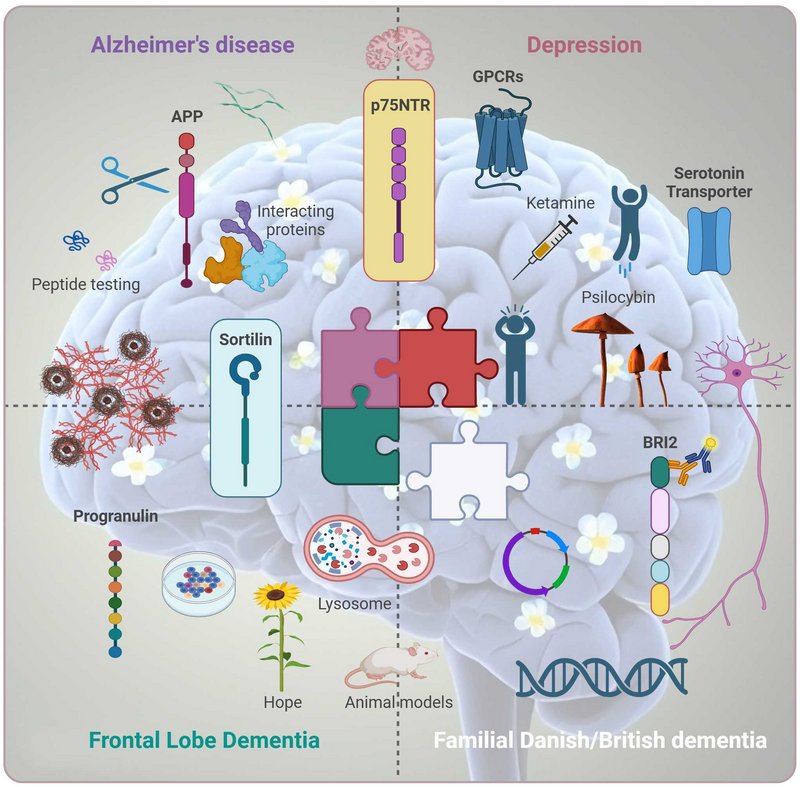
My research focuses on the molecular mechanisms that underlie neurodegenerative diseases and depression, with a particular interest in the converging pathways that may drive these conditions. Epidemiological and clinical studies have demonstrated that individuals with depression are at a significantly higher risk of developing dementia, including Alzheimer’s disease. This compelling link suggests that pathways involved in depression may accelerate the mechanisms that drive neurodegeneration.
To explore these connections, my lab investigates receptor dynamics as a central theme. We study processes such as receptor trafficking, oligomerization, protein-protein interactions, signaling, and sorting, which are fundamental to understanding how cellular communication and function are altered in both healthy and diseased states. Our approach begins at the molecular level, where we dissect these intricate processes, and extends to cellular and organismal contexts to bridge our findings to complex systems biology.
By integrating molecular, cellular, and whole-organism perspectives, we aim to shed light on the fundamental mechanisms underlying neurodegenerative diseases and depression, with the ultimate goal of identifying potential targets for therapeutic intervention.
Key Receptors in Focus
Our research places a strong emphasis on the p75 neurotrophin receptor (p75NTR), a multifunctional receptor involved in diverse cellular processes such as apoptosis, survival, and synaptic plasticity. p75NTR plays a pivotal role in neurodegenerative conditions, as its activation often promotes cell death under pathological conditions, including Alzheimer’s disease. Furthermore, p75NTR has been implicated in depression, suggesting it may serve as a critical molecular link between these two conditions. By studying its regulation and interactions with its co-receptors, we aim to uncover how p75NTR contributes to the shared pathways underlying depression and neurodegeneration.
Another receptor of significant interest is sortilin, a member of the Vps10p domain receptor family, which is a critical regulator of protein trafficking and signaling in neurons and other cell types. Sortilin interacts with several ligands, including neurotrophic factors, to influence processes such as neuronal survival, axonal growth, and synaptic function. Its ability to form complexes with p75NTR highlights its role in mediating neurotrophic signaling pathways that are disrupted in both depression and neurodegenerative diseases.
A key focus of our work is the role of sortilin in regulating progranulin, a neurotrophic factor essential for neuronal survival, inflammation modulation, and lysosomal function. Sortilin functions as a sorting receptor for progranulin, mediating its lysosomal degradation. Dysregulation of this pathway has been implicated in frontotemporal dementia (FTD) and other neurodegenerative disorders. By studying how sortilin modulates progranulin levels and trafficking, we aim to uncover mechanisms that may connect progranulin deficits to broader neurodegenerative and depressive pathways.
We also investigate the amyloid precursor protein (APP), a receptor-like molecule best known for its role in Alzheimer’s disease due to its cleavage into amyloid-beta peptides. Beyond its role in amyloid pathology, APP is involved in synaptic function and neuronal plasticity, which are crucial for mood regulation and cognitive function. Our research investigates the non-canonical proteolytic cleavage of APP, focusing on how this process is regulated by interactions with specific binding partners. By studying these alternative cleavage pathways, we aim to uncover novel mechanisms that influence APP processing, beyond the well-characterized amyloidogenic and non-amyloidogenic pathways.
Downstream Signaling of Rapid-Acting Antidepressants
In addition to receptor dynamics, we are exploring the downstream signaling pathways activated by rapid-acting antidepressants, such as psilocybin and ketamine. These compounds have shown remarkable efficacy in alleviating depressive symptoms, often within hours, by targeting previously underappreciated molecular and cellular mechanisms. Our work focuses on identifying how these drugs modulate signaling pathways related to synaptic plasticity, neurotrophic support, and cellular resilience.